At Dynamic Equilibrium?
At Dynamic Equilibrium
a) The reverse reaction stops
b) The forward reaction stops
c) Both forward and reverse reactions stop
d) Both Forward and reverse reactions continue at the same rate
Correct Answer (d)
Because in dynamic equilibrium A reaction that does not stop at equilibrium, the rate of forwarding and reverse reaction remains equal
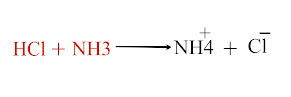
Comments
Post a Comment