Limitations of Arrhenius Concept
The limitations of Arrhenius concept are
Arrhenius concept is only limited to aqueous medium and dose not explain the acidity and basicity of the substance in non equeous medium
In Arrhenius concept acids are limited to hydrogen ion (H+) only and bases are limited to hydroxide ion (OH-). It cannot explain the acidic nature of carbon dioxide (CO2) and basic nature of ammonia (NH3)
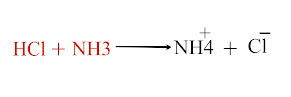
Comments
Post a Comment