Bronsted-Lowry Concept of Acids and Bases
The Danish chemist J. N. Bronsted and an English chemist T. M. Lowry independently expanded the Arrhenius acid and base definitions. According to this concept,
A Bronsted-Lowry acid is a substance (molecule or ion) that is a proton (H+) donor, whereas a Bronsted-Lowry base is a substance (molecule or ion) that is a proton (H+) acceptor.
For Example
Hydrochloric acid acts as a Bronsted-Lowry acid when it reacts with ammonia. In this example, (HCl) donates a proton and acts as an acid, while ammonia (NH3) accepts a proton to form an ammonium ion (NH4+) and serves as a base.
Water can also act as a Bronsted-Lowry acid. For example, The following reaction, in which the H2O molecule gives a proton to the NH3.
In another example, water acts as a Bronsted-Lowry base as it accepts a proton from HCI, which act as an acid.
Therefore, the water (H20) molecule acts both as an acid well as a base (amphoteric).
Some Arrhenius hydroxide bases, such as NaOH, are not Bronsted-Lowry bases. It is because these compounds are not proton acceptors whereas, the OH- ion produced in a solution is the Bronsted-Lowry base because it is the species that can accept a proton
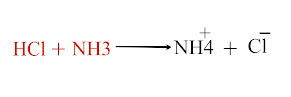

Comments
Post a Comment