Simple Definition of Arrhenius Acid and Base
Simple Definition of Arrhenius Acid and Base
Arrhenius Acids
An acid is a chemical substance that dissociates in an aqueous solution to give hydrogen ions (H+)
A substance such as HCl, H2SO4, HNO3, CH3COOH, HCN, etc, is acids because they ionize in an aqueous solution to produce H+ ions.
Arrhenius Base
Arrhenius base is a chemical substance that dissociates in an aqueous solution to give hydroxide ions (OH -)
The substances such as NaOH, KOH, NH4OH, Mg(OH)2, etc are bases because they ionize in aqueous solutions to provide OH- ions
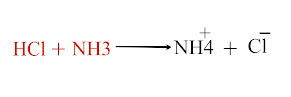
Comments
Post a Comment